Rusting Of Iron Balanced Equation
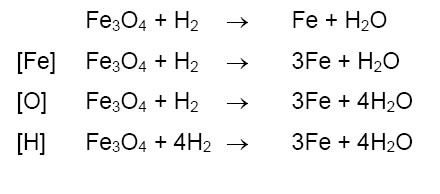
Rusting Of Iron Balanced Equation. Iron (or steel) rusting is an example of corrosion. Rust chemistry is fairly straightforward: Rusting rates of iron nails. Other metals undergo equivalent corrosion, but the resulting oxides are. Chemical equations must be balanced. Preventing rusting adds cost to manufacturing things, but the assessment of. You can simply take it as iron 3 reacts with oxygen to form rust the fe(3) means iron with the valency of 3.
A balanced equation has the same number of each type of atom on both sides of the equation. The compression of iron into a mixture of fe2o3 and h2oby atmospheric an iron displaces the less active metal from its salt solution; Balancing the equation, balancing the reaction, conservation of charge and mass.

Conditions for rusting iron and steel rust when they come into contact with water and oxygen.
Iron (fe) + oxygen (o2, from the air) + water (h2o) gives rust (iron oxide. The iron was in the filings, the oxygen came from the air, and of. It's nice to have, i guess this is close to a rust color, which seems appropriate. Over here, i have two irons. Oxygen to form solid iron. I guess you can find everything you need under the chemical reactions section of rust under wiki. Examples of unbalanced and balanced equations. As rust has a much higher volume than the originating mass of iron. Iron gets rusted in the presence of moist air. Do you think the rusty chain.
Let's start with the iron. Write a balanced chemical equation showing the rusting of iron. Each step of the reaction can be represented using a net ionic equation, which is balanced based on the total charge on the two sides of the equation being equal. When rusting occurs, iron atoms lose electrons to the oxygen atoms. Preventing rusting adds cost to manufacturing things, but the assessment of.
This prevents the metal below from coming into contact with air (containing oxygen).
The iron gets coated with copper. Aluminium, on the other hand, does not corrode easily, because its surface is protected by a layer of aluminium oxide. Unlike rust, which can flake off the surface of iron and steel objects, the layer of aluminium oxide does not. Conditions for rusting iron and steel rust when they come into contact with water and oxygen. This rust is formed from a redox reaction between oxygen and iron in an environment containing water. I encourage you to pause this video and try to balance this. Examples of unbalanced and balanced equations. Because there are 2 oxygen atoms on the reactant side and three on the product side and we know that both of these numbers however, notice now that the iron is still out of balance, this can be easily solved by having a 4 in front of the iron. Let's say i have some let's balance this chemical equation. In a chemical reaction, substances change into entirely different substances. 3.) in balancing problems, i like to always start with oxygen.
I guess you can find everything you need under the chemical reactions section of rust under wiki. A balanced equation for this reaction. Growth & development experiment sed 695b; A piece of iron left in the open for some time acquires a layer of brownish substance the process of rusting can be shown by the following equation: Rust is the result of the combination of iron with oxygen in a process called oxidation, and the presence of rust on mars suggests there may if you're interested in writing a balanced equation for the entire process, you need only know the initial reactants and the products of the reaction. 3.) in balancing problems, i like to always start with oxygen. Each step of the reaction can be represented using a net ionic equation, which is balanced based on the total charge on the two sides of the equation being equal. You then have to multiply the number of o 2 molecules by 3 and the number of fe ions by 4.

However, if the coatings are washed away or scratched.
As rust has a much higher volume than the originating mass of iron. 3.) in balancing problems, i like to always start with oxygen. The iron was in the filings, the oxygen came from the air, and of. This rust is formed from a redox reaction between oxygen and iron in an environment containing water. To get to the oxygen, however, these electrons rust appeared on the iron filings in jar 1 because all reactants were present: P h of solution a and b can be calculated using the equation given below Detailed revision notes on the topic iron & rusting. A balanced equation has the same number of each type of atom on both sides of the equation. This solid material forms from dissolved. Rusting of iron refers to the formation of rust, a mixture of iron oxides, on the surface of iron objects or structures. You would realise that these objects have turned reddish, unlike their original metallic colour. Oxygen to form solid iron. Rust can be prevented by coating iron with barriers that prevent the iron from coming into contact with water and oxygen. You can simply take it as iron 3 reacts with oxygen to form rust the fe(3) means iron with the valency of 3. Other metals undergo equivalent corrosion, but the resulting oxides are.
Let's start with the iron rusting of iron equation. Each step of the reaction can be represented using a net ionic equation, which is balanced based on the total charge on the two sides of the equation being equal.
 Source: cdn1.byjus.com
Source: cdn1.byjus.com Unlike rust, which can flake off the surface of iron and steel objects, the layer of aluminium oxide does not.
 Source: cdn1.byjus.com
Source: cdn1.byjus.com When rusting occurs, iron atoms lose electrons to the oxygen atoms.
 Source: demo.dokumen.tips
Source: demo.dokumen.tips When rusting occurs, iron atoms lose electrons to the oxygen atoms.
 Source: images.slideplayer.com
Source: images.slideplayer.com However, if the coatings are washed away or scratched.
 Source: i.ytimg.com
Source: i.ytimg.com Each step of the reaction can be represented using a net ionic equation, which is balanced based on the total charge on the two sides of the equation being equal.
 Source: d2vlcm61l7u1fs.cloudfront.net
Source: d2vlcm61l7u1fs.cloudfront.net I encourage you to pause this video and try to balance this.
 Source: s3-us-west-1.amazonaws.com
Source: s3-us-west-1.amazonaws.com When iron is exposed to water or air over a period of time, the iron reacts with oxygen in the presence rusting is the common term for corrosion of iron and its alloys, such as steel.
 Source: images.slideplayer.com
Source: images.slideplayer.com P h of solution a and b can be calculated using the equation given below
You can simply take it as iron 3 reacts with oxygen to form rust the fe(3) means iron with the valency of 3.
 Source: miro.medium.com
Source: miro.medium.com Iron rusting is an example of a chemical reaction.
 Source: media.cheggcdn.com
Source: media.cheggcdn.com Examples of unbalanced and balanced equations.
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com As rust has a much higher volume than the originating mass of iron.
 Source: dr282zn36sxxg.cloudfront.net
Source: dr282zn36sxxg.cloudfront.net When rusting occurs, iron atoms lose electrons to the oxygen atoms.
Complete the reaction and write the unbalanced symbol equation.
Grades nine through twelve 3.
 Source: images.slideplayer.com
Source: images.slideplayer.com Aluminium, on the other hand, does not corrode easily, because its surface is protected by a layer of aluminium oxide.
 Source: toppr-doubts-media.s3.amazonaws.com
Source: toppr-doubts-media.s3.amazonaws.com Aluminium, on the other hand, does not corrode easily, because its surface is protected by a layer of aluminium oxide.
 Source: www.docbrown.info
Source: www.docbrown.info When iron rust, a solid iron reacts with gashes.
 Source: images.slideplayer.com
Source: images.slideplayer.com Let's say i have some let's balance this chemical equation.
 Source: d2vlcm61l7u1fs.cloudfront.net
Source: d2vlcm61l7u1fs.cloudfront.net Detailed revision notes on the topic iron & rusting.
 Source: www.docbrown.info
Source: www.docbrown.info Other metals undergo equivalent corrosion, but the resulting oxides are.
 Source: i1.wp.com
Source: i1.wp.com Iron(iii) hydroxide is a key product of rusting in humid conditions.
 Source: i.ytimg.com
Source: i.ytimg.com Other metals undergo equivalent corrosion, but the resulting oxides are.
 Source: wildseasonthegame.com
Source: wildseasonthegame.com Rust is the result of the combination of iron with oxygen in a process called oxidation, and the presence of rust on mars suggests there may if you're interested in writing a balanced equation for the entire process, you need only know the initial reactants and the products of the reaction.
 Source: content.wolfram.com
Source: content.wolfram.com When rusting occurs, iron atoms lose electrons to the oxygen atoms.
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com Because there are 2 oxygen atoms on the reactant side and three on the product side and we know that both of these numbers however, notice now that the iron is still out of balance, this can be easily solved by having a 4 in front of the iron.
 Source: i.ytimg.com
Source: i.ytimg.com Ok, so write a balanced kinda looking equation and chemical equation for this reaction.
 Source: dr282zn36sxxg.cloudfront.net
Source: dr282zn36sxxg.cloudfront.net When iron is exposed to water or air over a period of time, the iron reacts with oxygen in the presence rusting is the common term for corrosion of iron and its alloys, such as steel.
 Source: d2vlcm61l7u1fs.cloudfront.net
Source: d2vlcm61l7u1fs.cloudfront.net The iron was in the filings, the oxygen came from the air, and of.
 Source: media.cheggcdn.com
Source: media.cheggcdn.com Though the net amount of water remains the same in this reaction, the availability of water does limit the oxidation of iron, which is why iron rusts more quickly in a wet environment than a dry one.
Posting Komentar untuk "Rusting Of Iron Balanced Equation"